Chondrocyte and Stem Cell Cartilage Repair
Achieving hyaline cartilage repair of cartilage defects is a biological challenge.
The goal of Autologous Chondrocyte Implantation is to produce articular cartilage repair with hyaline type cartilage without altering the integrity of the subchondral plate.
The procedure involves two stages, firstly, taking a biopsy of healthy cartilage from a non-weightbearing area of the knee from which chondrocytes are extracted and cell population expanded in a suitable environment.
The second stage is an open procedure when the cultured cells are implanted a few weeks later into the defect beneath a periosteal patch or alternative scaffold.
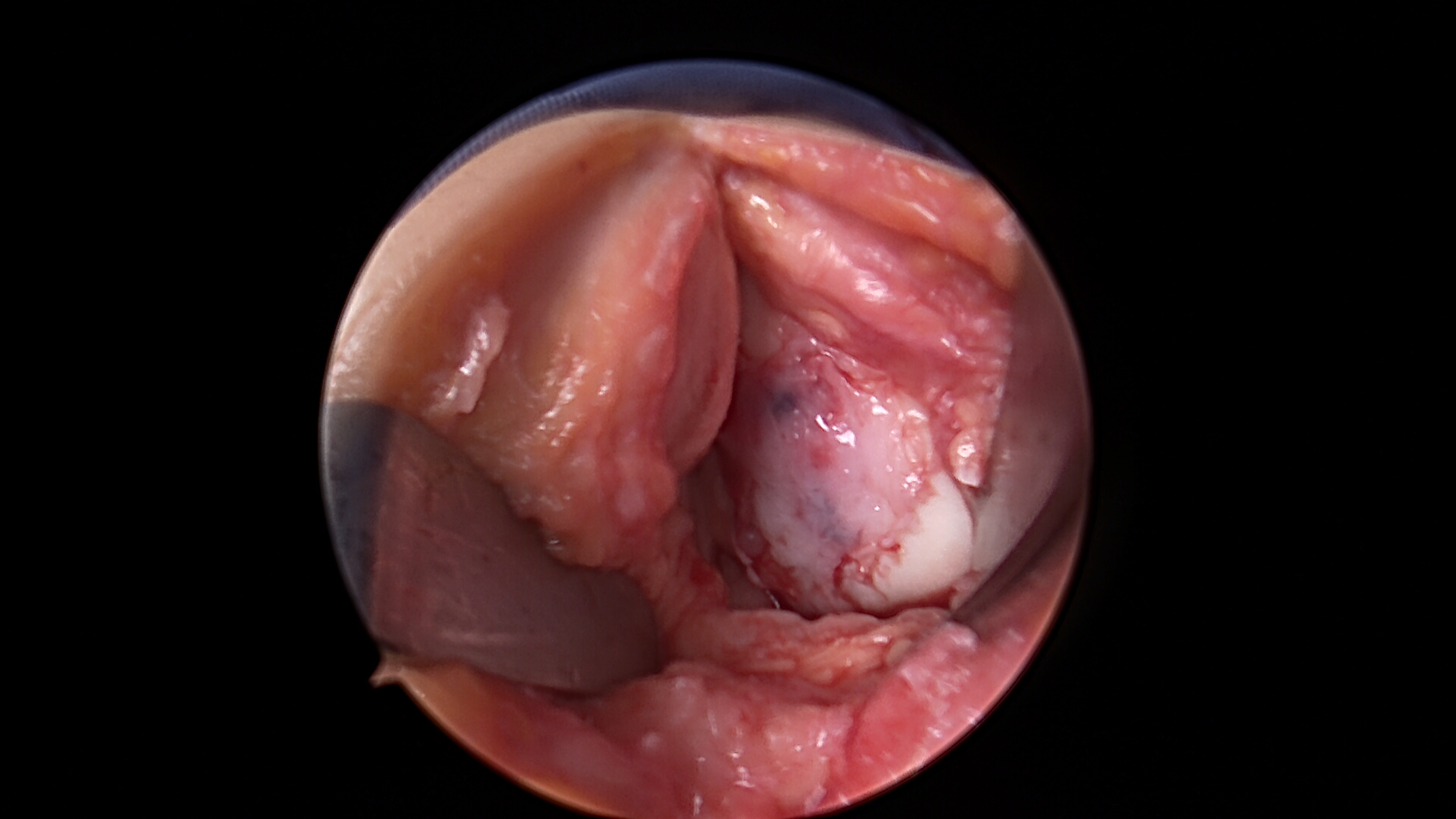
Cartilage Repair with Bone Marrow Stem cell and Scaffold
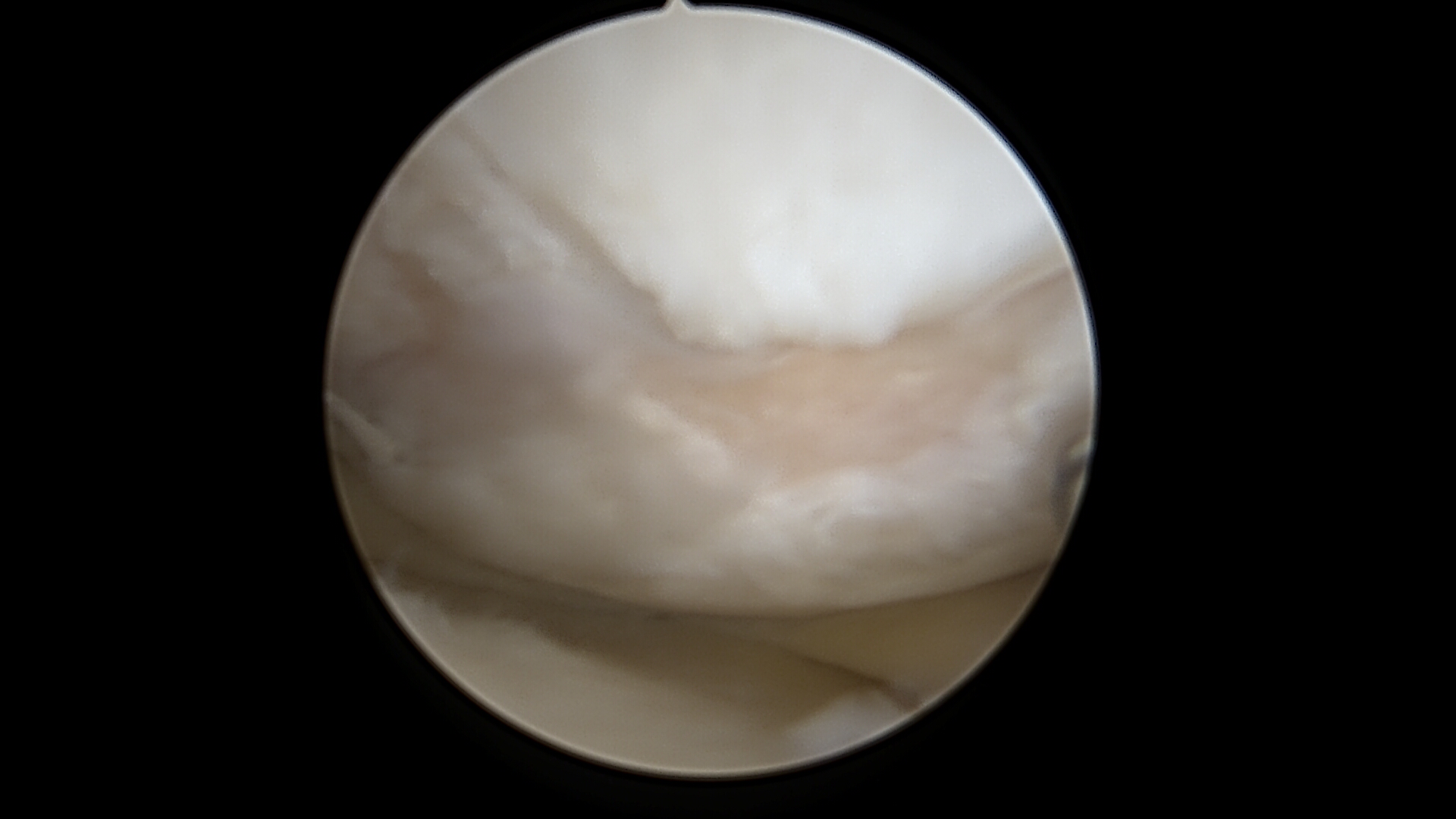
Cartilage Damage
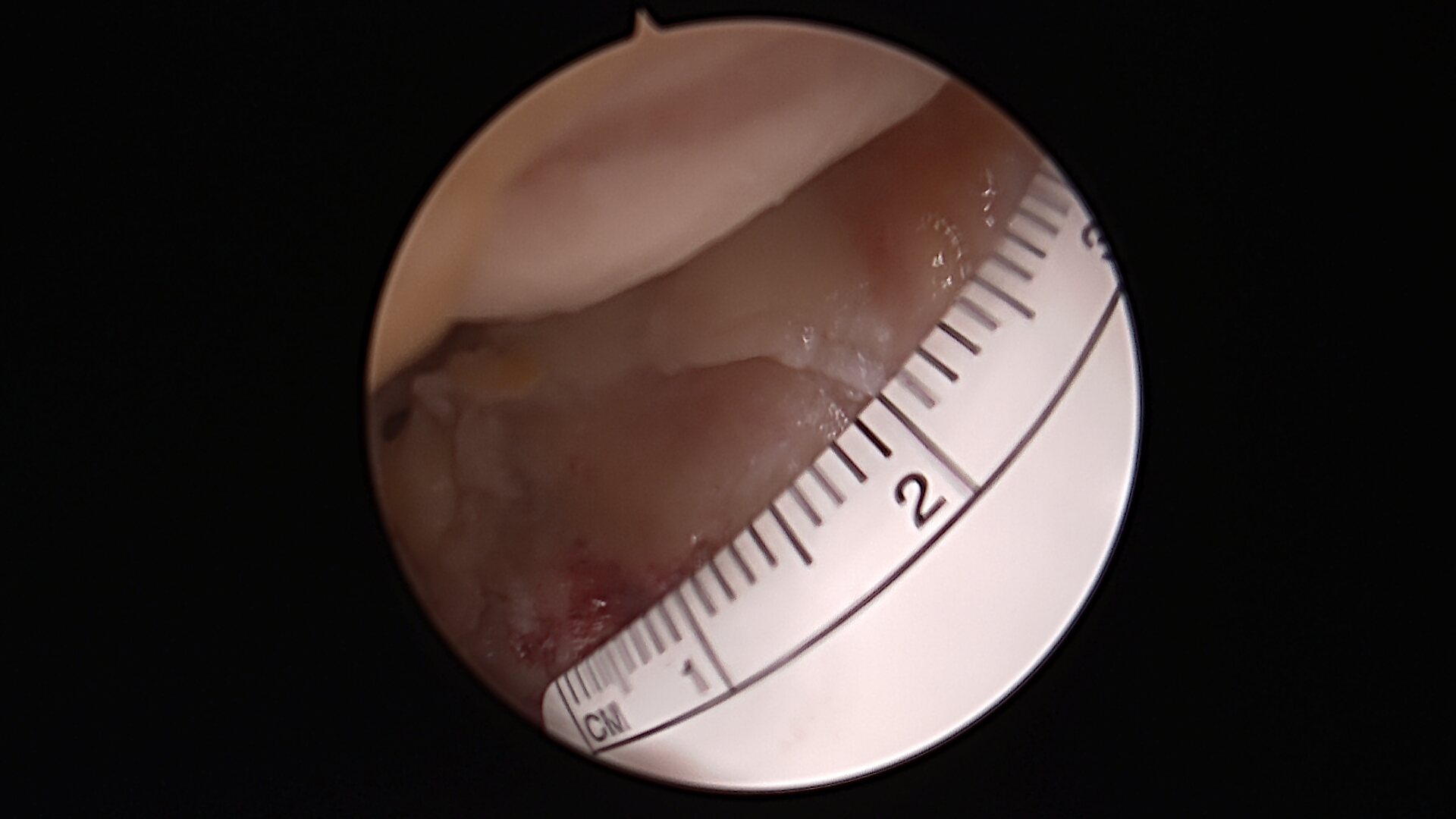
Preparing the Defect for Repair
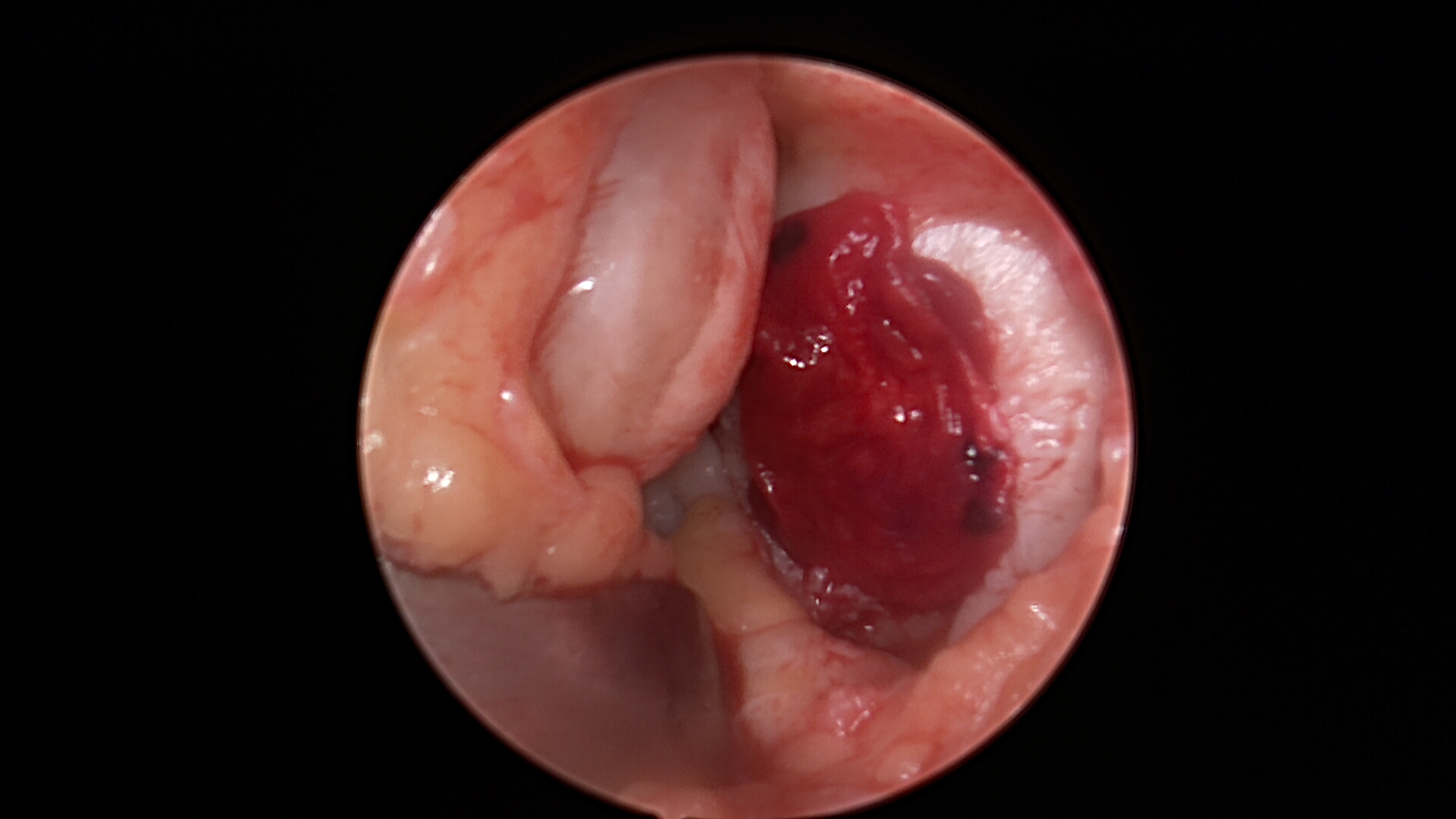
Stem Cell Paste Graft with Tissue Glue
Cells Covered with Scaffold Seeded with Cells
Autologous Chondrocyte Implantation
The main indication for ACI is failed primary treatment in a full thickness chondral lesion. Autologous Chondrocyte Implantation (ACI) is not indicated as routine first line treatment for chondral defects as there is paucity of good quality comparative cartilage repair studies, inadequate understanding of the natural history of chondral defects and controversy on the cost-effectiveness of ACI.
It is also important to study the importance of subchondral bone integrity to achieve long-term successful cartilage repair. Economic modelling using some assumptions about long-term outcomes that seem reasonable suggests that ACI would be cost-effective because it is more likely to produce hyaline cartilage, which is more likely to be durable and to prevent osteoarthritis in the longer term (e.g. 20 years).
The first steps to Autologous Chondrocyte Implantation started with studies on rabbits by O’Driscoll using periosteal grafting to treat chondral defects. Grande and Lars Peterson at the Department of Bioengineering, Hospital for Joint Diseases, New York, then refined a technique to culture autologous chondrocytes. In 1987 Lars Peterson and Brittberg, at the University of Gothenburg, began a human clinical trial to treat full-thickness chondral defects in the knee with Autologous Chondrocytes implanted behind a periosteal graft.
O’Driscoll SW, Keeley FW, Salter RB. The chondrogenic potential of free autogenous periosteal grafts for biologic resurfacing of major full-thickness defects in joint surfaces under the influence of continuous passive motion. An experimental investigation in the rabbit. J Bone Joint Surg Am. 1986;68:1017-1035.
Grande DA, Pitman MI, Peterson L, et al. The repair of experimentally produced defects in rabbit cartilage by autologous chondrocyte transplantation. J Orthop Res. 1989;7:208-218.
Brittberg M, Lindahl A, Nilsson A, et al: Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331:889, 1994.
First Generation ACI
The periosteum has osteogenic capacity, but it can also be used to promote cartilage formation in a chondrotrophic environment. Joint motion appeared to be one of the chondrogenesis-promoting factors. Isolated Periosteal grafting has been used experimentally to treat chondral defects since 1970s.
In first generation ACI, cultured Autologous Chondrocytes are injected beneath a periosteal flap sutured to the edges of the prepared chondral defect. This is performed in two stages- the first stage is an arthroscopic biopsy of normal articular cartilage from the supero-medial edge of the trochlea or the lateral edge of the intercondylar notch. A ring curette is used to harvest the graft and normally three small pieces of normal hyaline cartilage are sufficient. The chondrocytes are then cultured in-vitro in to about 10 million cells.
The second stage involves harvesting a periosteal graft from the proximal medial tibia. The defect in the knee joint is then exposed via a parapatellar arthrotomy. The defect is prepared to obtain stable healthy vertical edges and also removing the fibrocartilaginous cap covering the defect with a curette. A 15 scalpel blade is used to obtain vertical edges. Adrenaline soaked patties can help in achieving haemostasis. If there is more than 8mm bone defect, then bone grafting the bed may be required. The periosteal patch is sutured to the edges of the defect with the cambium layer facing underneath using 6-0 vicryl. The cells are then injected from the superior edge into the bed and the opening closed with fibrin glue. Remember to unload the compartment with a realignment osteotomy or Fulkerson osteotomy if required.
This is still the only technique currently approved by the FDA.
Second Generation ACI
Periosteal grafts have posed problems due to graft hypertrophy and also due to morbidity from the harvest site. To overcome this problem various bioabsorbable scaffolds are available, Larger defects can also be dealt with more easily. The scaffold is seeded with the Chondrocytes glued in to the defect with fibrin glue. The technique of preparation of the defect is the same for all types of ACIs. The scaffolds are glued using fibrin glue.
MACI produced by Genzyme uses a collagen I/III Scaffold
CaRes by Arthrokinetics uses a Collagen I gel
Hyalograft C by Fidia uses a Hyalurinic Acid Scaffold.
Third Generation ACI
The newer generation ACIs have tissue engineered cartilage graft in to a 3D structure to ensure uniform seeding of Chondrocytes in a 3D scaffold. The cells are with conditions to simulate loading. Neocart by Histogenics is currently on a Phase 2 trial.
ChondroCelect (TiGenix) contains phenotypically selected Chondrocytes and have been shown to have superior cartilage repair capacity. In a randomised controlled study compared with microfracture, Characterized Chondrocyte implantation was associated with repair tissue that is superior to that after microfracture.
Saris DB, Vanlauwe J et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008 Feb;36(2):235-46.
One Stage ACI
DeNovo ET (ISTO Technologies) is a cartilage implant, offering a one-step alternative to current cartilage repair and is undergoing Phase I/II trial. Designed to be offered as a product for the repair and regeneration of knee cartilage, DeNovo ET is derived from juvenile tissue and uses a protein-based adhesive in a one-step procedure.
Cartilage autograft implantation system (CAIS) by Depuy-Mitek uses minced autologous cartilage placed on a scaffold and is currently on a preclinical trial.
Evaluation of Cartilage repair and Histological scoring
Second look arthroscopic examination provides to determine if the lesion is filled and if the repair tissue is well integrated in the graft and with the surrounding cartilage is a valuable technique. Assessment of indentation stiffness gives an indication of the biomechanical properties of the repair tissue.
ICRS have provided a Visual Histological score for repair assessment. The criteria look at Surface, matrix, cell distribution, cell viability, subchondral bone and mineralization.
Outcomes of 1st Generation ACI
Peterson reported two to ten year results following first generation ACI treatment of Osteochondritis dissecans of the knee in fifty-eight patients. Patients underwent treatment between 1987 and 2000 and their mean age was 26.4 years. Twenty-two patients consented to arthroscopic second-look evaluation of graft integrity. Seven patients were less than eighteen years old. Thirty-five patients (60%) had juvenile-onset disease, and forty-eight patients (83%) had had a mean of 2.1 prior operations. The defect was located on the medial femoral condyle in thirty-nine patients and on the lateral femoral condyle in nineteen. The mean lesion size was 5.7 cm (2) (range, 1.5 to 12.0 cm (2) ), and the mean defect depth was 7.8 mm (range, 4 to 15 mm). After a mean duration of follow-up of 5.6 years, 91% of the patients had a good or excellent overall rating on the basis of a clinician evaluation and 93% had improvement on a patient self-assessment questionnaire. The Tegner, Lysholm, and Brittberg-Peterson VAS scores were all improved. The macroscopic quality of graft integrity averaged 11.2 on a 12-point scale, with only one graft having a score of more than 9 points. Two patients had a failure of treatment in the early postoperative period. Only one patient who had had a good or excellent rating at two years had a decline in clinical status at the time of the latest follow-up. Henderson reported 2-year treatment outcome of ACI in 53 patients (72 lesions) through clinical evaluation, MRI, second-look arthroscopy and biopsies. They showed improvement in mean subjective score from 37.6 (Preoperative) to 60.1 (24 months) and also improvement in Knee function levels (86% International Cartilage Repair Society (ICRS) III/IV to 66.6% I/II) from preoperative period to 24 months postimplantation. Objective IKDC score of A or B were observed in 88% preoperatively improving to 94.4% at 24 months post implantation. Transient deterioration in all these clinical scores was observed at 3 months before progressive improvement became evident. MRI studies demonstrated 75.3% with at least 50% defect fill, 46.3% with near normal signal, 68.1% with mild/no effusion and also 66.7% with mild/no underlying bone marrow oedema at 3 months. These values improved to 94.2%, 86.9%, 91.3% and 88.4%, respectively, at 12 months. At 24 months, further improvements to 97%, 97%, 95.6% and 92.6%, respectively, were observed. Second-look arthroscopy carried out in 22 knees (32 lesions) demonstrated all grafts to be normal/nearly normal based on the International Cartilage Repair Society (ICRS) visual repair assessment while core biopsies from 20 lesions demonstrated 13 grafts to have hyaline/hyaline-like tissue.
Cartilage Repair Registry (Browne CORR 2005)
This is a multicentre study with the implant procedures done at 40 centres between March 1995 and May 1996. Eligible study patients had autologous chondrocyte implantation for lesions of the distal femur. Modified 10-point scales of the Cincinnati knee rating system were used to measure outcomes assessments at baseline and at 5 years. There were 87 patients with an average 37 years of age and a mean total defect size of 4.9 cm2. Eighty-seven percent (87 of 100) of patients completed 5-year followup assessments. At least one prior cartilage repair procedure had failed in 70% of the patients. Patients who completed 5-year assessments revealed the following: 62 patients improved, six reported no change, and 19 worsened. Of the 62 patients who improved, the mean score for the modified perceived scale of overall condition increased (p < 0.0001) 4.1 points from baseline. At follow-up, 87 patients reported a mean improvement of 2.6 points in the overall condition score, including 62 with improved conditions, six with no change in condition, and 19 with worsened conditions. Of the 62 patients who improved, the mean overall condition score improved 4.1 points at followup.
Rosenberg and Minas showed encouraging results in a slightly older age group. Fifty-six patients 45 years of age or older were treated with ACI for a total of 117 defects. Overall, patients rated their outcomes at the latest available follow-up as 72% good or excellent, 15% fair, and 13% poor. Activity levels improved as reflected by the Modified Cincinnati Score, which increased from an average of 3.6 (“no sports possible”) to 5.9 (“some limitations with sports but I can participate”). Seventy-eight percent of patients felt improved by the surgery, and 81% would choose to undergo ACI again.
Second Generation ACI
In a prospective study, Steinwachs reported results of ACI with a collagen membrane using clinical examination and MRI. 63 patients (mean age, 34 years) with full-thickness chondral lesions of the knee underwent an autologous chondrocyte implantation and were evaluated preoperatively and at 6, 18, and 36 months after surgery. The chondrocyte suspension within the defect was covered with a type I/III collagen membrane. The ICRS and modified Cincinnati score showed significant improvement in all time intervals between preoperative and 6, 18, and 36 months after surgery. There was no significant difference in the final outcome between different defect localizations. The Pearson coefficient of correlation between clinical and MRI scores was 0.73 and significant at the 0.01 level. There was no patient with a symptomatic graft hypertrophy. Bentley compared the results of ACI-P and ACI-C. The first involves using a periosteal cover (ACI-P) and the second involves using a type I/III collagen membrane (ACI-C). A total of 68 patients with a mean age of 30.52 years with symptomatic articular cartilage defects were randomised to have either ACI-P (33 patients) or ACI-C (35 patients). The mean defect size was 4.54 cm2. All patients were followed up at 24 months. A clinical and functional assessment showed that 74% of patients had a good or excellent result following the ACI-C compared with 67% after the ACI-P at 2 years. Arthroscopy at 1 year also demonstrated similar results for both techniques. However, 36.4% of the ACI-P grafts required shaving for hypertrophy compared with none for the ACI-C grafts at 1 year. In patients with Osteochondritis dissecans, Bentley showed excellent or good clinical results in 27 of 37 patients. Arthroscopy at one year revealed International Cartilage Repair Society grades of 1 (excellent) or 2 (good) in 21 of 24 patients (87.5%) who consented to the procedure. Of the remaining three patients, two scored grade 3 (fair) and one had grade 4 (poor) repair. Of the 23 biopsies taken at one year, 11 (47.8%) showed either hyaline-like or a mixture of hyaline-like and fibrocartilage, and 12 demonstrated fibrocartilage (52.2%).
Comparative Studies
Knutsen (Norway) performed a prospective randomised trial between Microfracture and ACI with 40 patients in each group. The mean age was 32.2 years with 65% of the lesions being traumatic. Lesion size was between 2 and 10 cm2 and 89% involved the medial femoral condyle. They used International Cartilage Repair Society, Lysholm, Short Form-36 (SF-36), and Tegner forms to collect data and published 2 year and 5 year results. Younger and more active patients did better in both groups. There were two failures in the autologous chondrocyte implantation group and one in the microfracture group at 2 years and 23% failure rate (9 patients) by 5 years in both groups. Biopsy specimens were obtained from 84% and showed no significant differences. One-third of the patients in both groups had radiographic evidence of early osteoarthritis at five years. Patients with hyaline type repair tissue had no failures.
Saris (Netherlands) reported results from a multicentre randomised control trial. This compared microfracture with ACI using Characterised Chondrocytes ( Phenotypically selected chondrocytes with biological potency to form stable cartilage tissue in vivo). Patients had a single grade III to IV symptomatic cartilage defect of the femoral condyles. There were 13 surgeons at 13 centres with 61 patients in the microfracture group and 57 patients having CCI. Lesion size was between 1 to 5 cm2 and mean age was 33.9 years. Clinical outcomes using KOOS score at 12-18 months were similar. Biopsies were done and histology and histomorphometric analysis was done. Mean histomorphometric score, a measure of proteoglycan and collagen II, is considered a quantitative and objective measure of good structural regeneration of cartilage tissue. CCI had better repair tissue than Microfracture.
Bentley compared Mosaicplasty and ACI. A total of 100 consecutive patients with a mean age of 31.3 years (16 to 49) and with symptomatic lesions of the articular cartilage of the knee was randomised to have either mosaicplasty (42 patients) or ACI (58 patients). The mean lesion size was 4.66cm2. 46 lesions were traumatic; 71 lesions on the femoral condyles and 25 on the patella. At a mean follow-up of 19 months, 88% had excellent or good results after ACI compared with 69% after mosaicplasty. Arthroscopy at one year demonstrated excellent or good repairs in 82% after ACI and in 34% after mosaicplasty. All five patellar mosaicplasties failed.
Horas (Giessen, Germany) reported two-year outcomes from a prospective randomised study in forty patients with a traumatic articular cartilage lesion of the femoral condyle treated with either OATS or ACI. The OATS was performed mini-open and ACI was first generation (ACI-P). Mean patient age was 33.4 years with mean lesion size 3.75 cm2. There was no significant difference in the Lysholm and Tegner Activity scores. OATS showed hyaline cartilage repair not integrated with surrounding normal cartilage whilst ACI showed mainly fibrocartilaginous repair.
Visna in a randomised study compared MACI and Abrasion Chondroplasty. There were 50 patients with mean age 30.8 years and lesion size between 2-10 cm2. 73% were femoral condyle lesions, 18% patella and 8% tibial plateau. Results at 12 months both in Lysholm and IKDC were better for ACI.
Fu compared Debridement and ACI. A retrospective analysis was performed on prospectively collected data. 58 patients were identified for each group who had similar demographics and chondral lesions. Follow-up outcome assessments were completed by 54 autologous chondrocyte implantation patients and 42 debridement patients. Eighty-one percent of the autologous chondrocyte implantation patients and 60% of the debridement patients reported median improvements of 5 points and 2 points, respectively, in the overall condition score. Patients who received autologous chondrocyte implantations obtained higher levels of knee function and had greater relief from pain and swelling at 3 years. ACI patients had more reoperations and also had multiple previous procedures.
ACI for Patellofemoral Joint
Retropatellar cartilage defects treated with autologous chondrocyte implantation (ACI) are still associated with inferior clinical outcome compared to defects being located on the femoral condyles. Minas in a prospective study showed the results for ACI in the patellofemoral articulation. There were 45 patients who reached a minimum follow-up of 2 years (range, 2-7 years). The average resurfacing per knee (n = 45) was 10.45 cm2. There were eight failures (18%) as a result of a patella or trochlea failure. Seventy-one percent of patients rated their outcomes as good or excellent. ACI for patellofemoral joint appears to have better results when combined with patella realignment osteotomy even if the preoperative tracking was normal (Henderson). The patella has greater shear forces and the patellar ridge has a particularly important role in the reduction of these shearing forces. Niemeyer describes a ‘double eye’ technique to retain the patella ridge and reported excellent or good results in 9 out of 11 patients.
Complications of ACI
Typical major complications are:
- hypertrophy of the transplant- upto 30.8%
- disturbed fusion of the regenerative cartilage and the healthy surrounding cartilage (23%)
- insufficient regenerative cartilage (17%)
- delamination.(17%)
In a series of 309 patients who had ACI between 2001 and 2006, Niemier showed that the rate of patients requiring revision surgery was highest in the periosteum group (26.9%), followed by the BioSeed-C group (14.6%) and the Chondrogide-covered ACI group (12.1%). No influence of patient age and defect size on the incidence of revision surgery was found. The incidence of hypertrophy of the transplant was highest in the periosteum-covered group (15.4%) and lowest in the Chondrogide-covered ACI group (1.9%).
References
- Jones DG, Peterson L. Autologous chondrocyte implantation. Instr Course Lect. 2007;56:429-45
- Krishnan SP, Skinner JA, Bartlett W, Carrington RW, Flanagan AM, Briggs TW, Bentley G. Who is the ideal candidate for autologous chondrocyte implantation? J Bone Joint Surg Br. 2006 Jan;88(1):61-4.
- Ritsilä VA, Santavirta S, Alhopuro S et al. Periosteal and perichondral grafting in reconstructive surgery. Clin Orthop Relat Res. 1994 May;(302):259-65.
- Peterson L, Minas T, Brittberg M, Lindahl A.Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am. 2003;85-A Suppl 2:17-24
- Marlovits S, Striessnig G, Kutscha-Lissberg F et al. Early postoperative adherence of matrix-induced autologous chondrocyte implantation for the treatment of full-thickness cartilage defects of the femoral condyle. Knee Surg Sports Traumatol Arthrosc. 2005 Sep;13(6):451-7
- Gille J, Meisner U, Ehlers EM, Müller A, Russlies M, Behrens P. Migration pattern, morphology and viability of cells suspended in or sealed with fibrin glue: a histomorphologic study. Tissue Cell. 2005 Oct;37(5):339-48Steinwachs M, Kreuz PC. Autologous chondrocyte implantation in chondral defects of the knee with a type I/III collagen membrane: a prospective study with a 3-year follow-up.Arthroscopy. 2007 Apr;23(4):381-7
- Henderson I, Francisco R, Oakes B, Cameron J. Autologous chondrocyte implantation for treatment of focal chondral defects of the knee–a clinical, arthroscopic, MRI and histologic evaluation at 2 years. Knee. 2005 Jun;12(3):209-16. Epub 2004 Nov 14
- Gooding CR, Bartlett W, Bentley G, Skinner JA, Carrington R, Flanagan A. A prospective, randomised study comparing two techniques of autologous chondrocyte implantation for osteochondral defects in the knee: Periosteum covered versus type I/III collagen covered. Knee. 2006 Jun;13(3):203-10.
- Bartlett W, Skinner JA, Gooding CR, Carrington RW, Flanagan AM, Briggs TW, Bentley G. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005 May;87(5):640-5
Outcomes
- Brittberg M. Autologous chondrocyte implantation–technique and long-term follow-up. Injury. 2008 Apr;39 Suppl 1:S40-9
- Rue JP, Yanke AB, Busam ML, McNickle AG, Cole BJ. Prospective evaluation of concurrent meniscus transplantation and articular cartilage repair: minimum 2-year follow-up. Am J Sports Med. 2008 Sep;36(9):1770-8.
- Behrens P, Bitter T, Kurz B, Russlies M. Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI)–5-year follow-up. Knee. 2006 Jun;13(3):194-202.
- Steinwachs M, Kreuz PC. Autologous chondrocyte implantation in chondral defects of the knee with a type I/III collagen membrane: a prospective study with a 3-year follow-up. Arthroscopy. 2007 Apr;23(4):381-7.
- Clar C, Cummins E, McIntyre L, Thomas S, Lamb J, Bain L, Jobanputra P, Waugh N. Clinical and cost-effectiveness of autologous chondrocyte implantation for cartilage defects in knee joints: systematic review and economic evaluation. Health Technol Assess. 2005 Dec;9(47):iii-iv, ix-x, 1-82.
- Bartlett W, Skinner JA, Gooding CR, Carrington RW, Flanagan AM, Briggs TW, Bentley G. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005 May;87(5):640-5.
- Browne JE, Anderson AF, Arciero R, Mandelbaum B, Moseley JB Jr, Micheli LJ, Fu F, Erggelet C. Clinical outcome of autologous chondrocyte implantation at 5 years in US subjects. Clin Orthop Relat Res. 2005 Jul;(436):237-45.
- Mandelbaum B, Browne JE, Fu F, Micheli LJ, Moseley JB Jr, Erggelet C, Anderson AF. Treatment outcomes of autologous chondrocyte implantation for full-thickness articular cartilage defects of the trochlea. Am J Sports Med. 2007 Jun;35(6):915-21
- Krishnan SP, Skinner JA, Carrington RW, Flanagan AM, Briggs TW, Bentley G. Collagen-covered autologous chondrocyte implantation for osteochondritis dissecans of the knee: two- to seven-year results. J Bone Joint Surg Br. 2006 Feb;88(2):203-5.
- Selmi TA, Verdonk P, Chambat P, Dubrana F, Potel JF, Barnouin L, Neyret P. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: outcome at two years. J Bone Joint Surg Br. 2008 May;90(5):597-604.
- Henderson I, Francisco R, Oakes B, Cameron J. Autologous chondrocyte implantation for treatment of focal chondral defects of the knee–a clinical, arthroscopic, MRI and histologic evaluation at 2 years. Knee. 2005 Jun;12(3):209-16.
- Magnussen RA, Dunn WR, Carey JL, Spindler KP. Treatment of focal articular cartilage defects in the knee: a systematic review. Clin Orthop Relat Res. 2008 Apr;466(4):952-62.
Comparative Studies
- Knutsen G, Drogset JO, Engebretsen L et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007 Oct;89(10):2105-12.
- Knutsen G, Engebretsen L et al. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004 Mar;86-A(3):455-64.
- Saris DB, Vanlauwe J, Victor J, Haspl M et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008 Feb;36(2):235-46
- Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am. 2003 Feb;85-A(2):185-92.
- Bentley G, Biant LC, Carrington Rwet al. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003 Mar;85(2):223-30
- Visna P, Pasa L, Cizmár I, Hart R, Hoch J. Treatment of deep cartilage defects of the knee using autologous chondrograft transplantation and by abrasive techniques–a randomized controlled study. Acta Chir Belg. 2004 Nov-Dec;104(6):709-14.
- Fu FH, Zurakowski D, Browne JE, Mandelbaum B, Erggelet C, Moseley JB Jr, Anderson AF, Micheli LJ. Autologous chondrocyte implantation versus debridement for treatment of full-thickness chondral defects of the knee: an observational cohort study with 3-year follow-up. Am J Sports Med. 2005 Nov;33(11):1658-66
- Patellofemoral ACI and ACI complications
- Minas T, Bryant T. The role of autologous chondrocyte implantation in the patellofemoral joint. Clin Orthop Relat Res. 2005 Jul;(436):30-9.
- Farr J. Autologous chondrocyte implantation improves patellofemoral cartilage treatment outcomes. Clin Orthop Relat Res. 2007 Oct;463:187-94.
- Henderson IJ, Lavigne P. Periosteal autologous chondrocyte implantation for patellar chondral defect in patients with normal and abnormal patellar tracking. Knee. 2006 Aug;13(4):274-9.
- Niemeyer P, Kreuz PC, Steinwachs M, Köstler W, Mehlhorn A, Kraft N, Südkamp NP. Technical note: the “double eye” technique as a modification of autologous chondrocyte implantation for the treatment of retropatellar cartilage defects. Knee Surg Sports Traumatol Arthrosc. 2007 Dec;15(12):1461-8
- Vasara AI, Nieminen MT, Jurvelin JS, Peterson L, Lindahl A, Kiviranta I. Indentation stiffness of repair tissue after autologous chondrocyte transplantation. Clin Orthop Relat Res. 2005 Apr;(433):233-42.
- Niemeyer P, Pestka JM, Kreuz PC, Erggelet C, Schmal H, Suedkamp NP, Steinwachs M. Characteristic Complications After Autologous Chondrocyte Implantation for Cartilage Defects of the Knee Joint. Am J Sports Med. 2008 Sep 18.
- Rosenberger RE, Gomoll AH, Bryant T, Minas T. Repair of Large Chondral Defects of the Knee With Autologous Chondrocyte Implantation in Patients 45 Years or Older. Am J Sports Med. 2008 Aug 25.